Job Ad new
![]() Last modified: 15/04/2002 by
Last modified: 15/04/2002 by
Research Topics
- In brief...
- Why axon?
- The Slow Wallerian degeneration mouse (C57Bl/Wlds); the Wld gene
- The protective mechanism
- The effect of Wld on disease
- Project ideas-free to a good home.
A Wld transgene protects distal axons for 14 days following nerve transection!!!
![]()
We have identified
a mutant mouse gene with the unique property of delaying Wallerian degeneration
of cut axons for several weeks (Mack
et al., 2001). The
protective mechanism appears to involve altered ubiquitination or NAD+
metabolism and may be indirect, thus indicating the existence of other such
genes. Our new methods to track the
inheritance of the mutant gene (Mi
et al., in press) have been used by collaborating laboratories to
show that the mutant gene protects axons in models of human neurodegenerative
diseases (Samsam and Martini, in preparation; Ferri and Kato, personal
communication). Identification of
one of the corresponding human genes (Fernando
et al., in press) will allow analysis of any role it may play in
human neurological disorders.
 Back to Top
Back to Top
![]()
Why axons?
Why
keep a neuron cell body alive in a central nervous system disease if it doesn't have
an axon ? Why let a peripheral axon
degenerate during a temporary exposure to a toxin and have it reinnervate the
wrong target ? What can we do to
protect those axons that are not transected by a spinal cord injury, but die
anyway ?
Very
much more is known about how to keep neuronal cell bodies alive than about how
to keep axons alive. Wallerian
degeneration, the degeneration of the distal segment of an injured axon, is
still poorly understood 150 years after it was first described.
This is in spite of increasing awareness of the importance of axon
degeneration in human neurodegenerative disorders as diverse as amyotrophic
lateral sclerosis, multiple sclerosis, traumatic brain injury, spinal cord
injury, Huntington's disease and glaucoma.
Effective preservation of the neuronal cell body is likely to be just one
part of the solution.
 Back to Top
Back to Top
![]()
The Slow Wallerian degeneration mouse(C57BL/Wlds); the Wld gene
The Slow Wallerian degeneration mouse, C57BL/WldS,
carries a spontaneous dominant mutation that delays Wallerian degeneration more
than 10-fold. The mouse is
developmentally normal and completely healthy.
Following the fortuitous identification of this
mutant in 1989, characterisation of the phenotype by Hugh Perry and Michael
Brown at the University of Oxford showed that the protective effect is intrinsic
to axons and widely expressed in the central and peripheral nervous system.
Hugh and others proposed that, since axon degeneration is can be
genetically regulated, it may be an active process akin to apoptosis rather than
a passive process, as previously assumed. The
Wld gene was mapped in collaboration with Mary Lyon to distal mouse
chromosome 4 (Lyon et al.,
1993) and further genetic and physical mapping led to
the identification of an 85 kb triplicated genomic segment in this region
(Coleman et al.,
1998).
The
Wlds triplication and its genes
Within the triplicated region we
identified a candidate chimeric gene, which we showed to encode an in-frame
fusion protein, now known to consist of the ubiquitination factor Ube4b and a
key enzyme of NAD synthesis, Nmnat (Conforti et al.,
2000).
We have now proven that the chimeric gene is the Wld gene by
reproducing the slow Wallerian degeneration phenotype
in four lines of transgenic mice (Mack et al.,
2001).
Protected
cytoskeleton in lesioned transgenic axons
Protected
motor axon terminals http://www.dns.ed.ac.uk/rrrweb/CellImgs.htm
 Back to Top
Back to Top
![]()
The protective mechanism
We have begun characterising the
protective mechanism and made the surprising observation that the Wld protein is
predominantly located within the nucleus.
This suggests that other factor(s)
mediate the protective effect on the axon and leads us to propose the existence
of other genes that could have a related effect to Wld. In collaboration with Professor Giulio Magni (Ancona)
we have found that the Wld protein possesses enzyme activity for NAD+
synthesis,
although it remains to be determined whether this, or altered ubiquitination, underlies the protective effect. Finally, we have discovered that the protective effect is highly dose-dependent, a finding which has important implication for attempts unto use Wld alter the course of neurodegenerative disease in animal models and, if appropriate, in humans.
 Back to Top
Back to Top
![]()
The effect of Wld on disease
There's nothing more useless than a cut
axon ! So one line of
reasoning goes that having a Wld gene is bad news because it delays the
removal of a useless bag of cytoplasm. If
you believe that Wld is of no possible practical use, stop reading now.
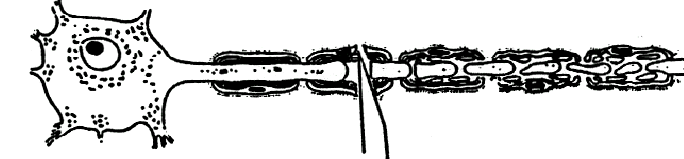
Alternatively, if the protective
effect of the Wld gene is strong enough to keep cut axons alive for two
weeks, just what else could it do ? Could
it protect sick or injured non-transected axons, maybe for an even longer time
period ? We and others have set out
to answer this question.
One way to do this is to cross WldS
mice with mice that have a neurodegenerative disease. However, this is not as simple as it sounds as often you need
to track the inheritance of the mutation through three generations, especially
if you want to make it homozygous. Thus,
we have developed genotyping methods for WldS (Mi et al., in
press).
Using these methods, the groups of Rudolf Martini (Würzburg) and Ann Kato (Geneva) have made some exciting observations. The WldS mutation significantly prevents axon loss that occurs due to dysmyelination in the myelin protein zero knockout mutant, and that this leads to functional improvement (Samsam and Martini, in preparation) and it improves the phenotype in the mouse motoneuron disease mutant pmn (Ferri and Kato, unpublished data). In our own laboratory we are crossing WldS mice with gracile axonal dystrophy mice, which have a dying-back axonopathy.
Dr. Shama Fernando (Oxford) is studying the human homologues of
the two component Wld genes, and has developed Single Nucleotide Polymorphisms to test
for allelic association with human
neurodegenerative diseases.
Sequence
alignment of human and mouse Nmnat
 Back to Top
Back to Top
![]()
Project ideas-Free to a good home.
We would actively encourage and help anyone who is
interested to study the following questions.
If you are interested, please get in touch with Michael (Michael.Coleman@Uni-Koeln.de)
Analysis of the effect of the Wld gene on a wide range
of neurodegenerative diseases.
The following are ongoing studies in ours or other labs:
If you are interested in studying Wlds in other models, we
would be happy to help.
- myelin related peripheral axon loss
- motor neuron disease
- gracile axonal dystrophy
- glaucoma
- vincristine toxicity
- BPAU toxicity
- acrylamide toxicity
- spinal cord injury
The effect of the Wld gene on other cell types:
the protective effect of the Wld gene has only been studied in neurons,
but the protein expression pattern in the WldS mouse is much wider,
including skeletal muscle, spleen, lung and liver. In our transgenic mice (β-actin promoter), it is likely to be
wider still. Can it protect your
favourite cell type from something?
Human Disease: do you know a human neurodegenerative disease mapping to chromosome 1p36, especially one involving axon loss?
Retinol binding protein7: Rbp7 is triplicated and over expressed in the Wlds mouse, but it is outside the scope of our work. It is expressed in adipose tissue and mammary gland at a high level and elsewhere at low levels. Can the Wlds mouse help to find out what it does?
 Back to Top
Back to Top
![]()