Neues
Research Report
The Reduction of Rare-Earth Metal Halides with Unlike Metals -
Wöhler's Metallothermic Reduction
Dedicated to Professor Rudolf Hoppe on the Occasion of his 85th Birthday,
in Admiration for his Great Impact on Solid State Chemistry
Abstract
Rare-earth halides may be reduced by rare-earth metals (conproportionation)
and, as an alternative, by unlike metals such as alkali or alkaline-earth
metals, a route first established for the production of rare-earth metals.
It has great power for exploratory research subject to enhanced reactivity
at lower temperatures and the formation of alkali halide flux for crystal
growth. A large number of new compounds, ternary and higher, salt-like
and (semi-)metallic including interstitially stabilized cluster compounds
has been synthesized and characterized during the last decades.
Gerd Meyer, Z. Anorg. Allg. Chem. 2007, 633, 2537-2552.
Research Report
The Oxidation of Metals with Liebig Acids
Dedicated to Professor Dieter Naumann, Colleague and Friend, on the Occasion
of his 65th Birthday
Abstract
In Liebig's definition, an acid is a compound which contains one or more
hydrogen atoms which may be substituted by metal atoms. Hence, reactions
of Liebig acids in substance, excluding water or any other solvent, with
non-noble metals yield salts and release hydrogen. In this sense, not
only the classical mineral acids such as sulfuric or nitric acid, respectively,
are Liebig acids. Rather, there is a large variety of organic compounds
with, for example, HO- or HN-functions with acid constants that allow
for substitution of the hydrogen atoms by a metal atom. Simple covalent
hydrides like water and ammonia or even methane may also act as Liebig
acids with conditions properly chosen. The ammonium ion, (NH4)+,
represents a special case as it is available in a large variety of salts
and may react as an acid/oxidant or as a (base)/reductant and is also
a pseudo alkali-metal cation. The versatility of the ammonium ion is reviewed
with special emphasis to its ability to function as a Liebig acid, i.e.,
reactions of, especially, ammonium halides with non-noble metals.
Gerd Meyer, Z. Anorg. Allg. Chem. 2008, 634, 201-222.
Superbulky Ligands and Trapped Electrons: New Perspectives in Divalent Lanthanide Chemistry
Spectacular developments in recent years, especially the discovery of an anionic complex of divalent lanthanum, in which the electron is trapped in a localized 5d1 SOMO (see structure of the anion of [K([2.2.2]crypt)][LaCp''3] (Cp''=1,3-(SiMe3)2C5H3); La red, C gray, Si black), have brought new impetus to the solution chemistry of divalent lanthanides.
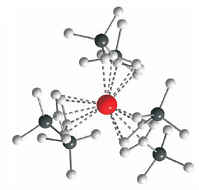
Gerd Meyer, Angew. Chem. Int. Ed. 2008, 47, xxxx-xxxx; Angew. Chem. 2008, 120, xxxx-xxxx.
Chains of face-sharing osmium-centered cubes and
square antiprisms
in the crystal structure of {Os3Sc12}Br16Sc
with 15 cluster-based electrons and two electrons consumed by an Os-Os
bond
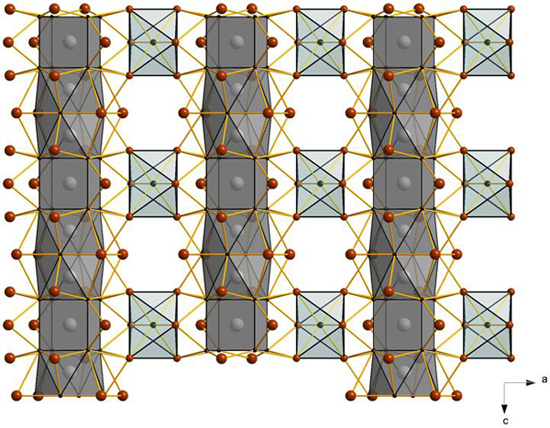
Sina Zimmermann, Gerd Meyer, unpublished 2008
RuHo5I7: An Intergrowth of {Ru4Ho16}I20 and {Ho4}I8 ?
Kathrin Daub, Gerd Meyer, unpublished 2008.
Tantalum(IV) Iodide: Forgotten and Resurrected
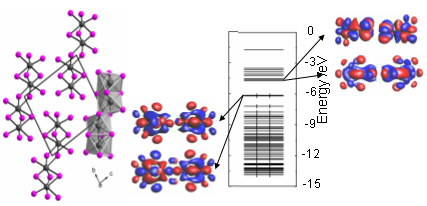
Gerd Meyer, Rafal Wiglusz, Ingo Pantenburg, Anja-Verena Mudring, Z. Anorg. Allg. Chem., 2008, 634, 825-828.
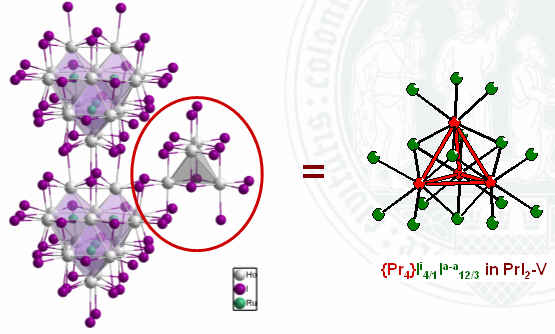
Seven-coordinate ruthenium atoms sequestered
in praseodymium clusters in the chloride {RuPr3}Cl3
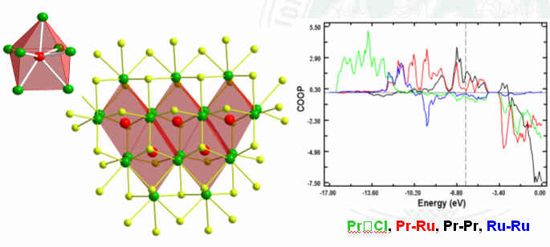
Nina Herzmann, Anja-Verena Mudring, Gerd Meyer, Inorg. Chem. 2008.
Facile synthesis of an iodine inclusion compound.
Molecular iodine in the anionic chains [I2@Pd2I6]2-
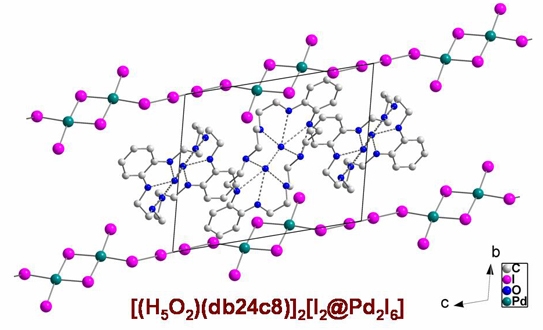
Christine Walbaum, Ingo Pantenburg, Gerd Meyer, Inorg. Chem. 2008.
Iodine molecules included in the crystal structure
of dibenzo-24-crown-8
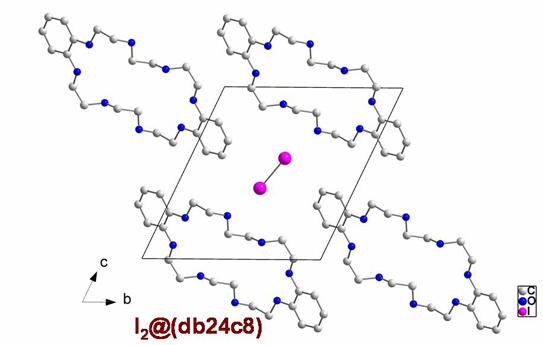
Christine Walbaum, Ingo Pantenburg, Gerd Meyer, unpublished 2008.
Iodine molecules attached to CdI2 or
HgI2 molecules
sequestered in benzo-18-crown-6

Ralph Striebinger, Christine Walbaum, Ingo Pantenburg, Gerd Meyer,
Z. Anorg. Allg. Chem. 2008.
![]()
Prof. Dr. Gerd Meyer - www.gerdmeyer.de
| Universität zu Köln
| zum Seitenanfang
